Understanding the Construction & Working Principle of Lithium-Ion Batteries
In our modern, tech-driven world, lithium-ion batteries are the lifeblood of mobile devices, electric vehicles (EVs), renewable energy storage, and countless other applications. Yet, despite their ubiquity, many still wonder: What is a lithium-ion battery and how does it work? This blog post aims to demystify the construction and working of lithium-ion batteries, providing a comprehensive breakdown of their structure, chemical operation, and applications. Whether you’re a student, engineer, EV enthusiast, or just curious, by the end of this guide, you’ll have a clear understanding of how lithium-ion batteries work and why they are the preferred energy storage solution of our time.
What is a Lithium-Ion Battery?
BA lithium-ion battery (Li-ion battery) is a type of rechargeable battery that uses lithium ions to store and release energy. Lithium, the lightest of all metals, offers high energy density, making it ideal for compact, lightweight power sources.So, what is Li-ion battery technology all about? At its core, a lithium-ion battery converts chemical energy into electrical energy through electrochemical reactions involving lithium ions moving between two electrodes: the anode and the cathode.
Key Features of Lithium-Ion Batteries
1. High Energy Density
One of the most significant advantages of lithium-ion batteries is their high energy density. This means they can store a large amount of energy relative to their weight and size. In practical terms:
- A compact lithium-ion battery can power a smartphone for an entire day or more.
- In electric vehicles (EVs), high energy density translates to longer driving ranges without increasing the battery’s size or weight excessively.
This feature is crucial in applications where space and weight are limited but performance is non-negotiable.
2. Low Self-Discharge Rate
All batteries gradually lose their charge when not in use. However, lithium-ion batteries boast a very low self-discharge rate, typically around 1.5–2% per month. This is significantly lower than other rechargeable batteries like NiMH (Nickel-Metal Hydride) or NiCd (Nickel-Cadmium), which can lose up to 20% per month.
Because of this, lithium-ion batteries retain power much longer when idle, making them ideal for emergency backup systems, portable electronics, and devices that aren’t used daily.
3. Lightweight and Compact
Lithium is the lightest metal on the periodic table, and lithium-ion batteries leverage this characteristic to be both lightweight and compact. Compared to traditional battery chemistries (like lead-acid or NiCd), lithium-ion batteries deliver:
- More power per kilogram, enabling sleek designs in gadgets
- Reduced overall device weight, which is critical for smartphones, laptops, drones, and especially electric vehicles where weight impacts performance and efficiency
This is a primary reason why lithium-ion batteries are the top choice in aerospace, automotive, and portable electronics.
4. Fast Charging Capabilities
Modern consumers demand quick power solutions. Lithium-ion batteries support fast charging, which has become a defining feature in everything from smartphones to EVs.
- In smartphones, fast charging tech can deliver 50–70% charge in under 30 minutes.
- In electric vehicles, DC fast chargers can restore 80% of the battery’s charge in 20–40 minutes.
This fast turnaround time is critical for reducing device downtime and improving user experience across all sectors.
5. No Memory Effect
The memory effect is a phenomenon where rechargeable batteries lose their maximum energy capacity when repeatedly recharged after being only partially discharged. Common in older chemistries like NiCd, it results in decreased battery life and performance over time.
Lithium-ion batteries, however, do not suffer from the memory effect. This means:
- You can top up your battery whenever it’s convenient — no need to fully discharge.
- There’s no long-term harm to capacity from frequent partial charges.
This flexibility makes lithium-ion batteries far more user-friendly and efficient in real-world usage.
6. Longer Lifespan
Compared to traditional rechargeable batteries, lithium-ion batteries have a significantly longer cycle life. On average, they can last:
- 500 to 1,000+ full charge-discharge cycles in consumer electronics
- Up to 2,000 cycles or more in EVs and advanced energy storage systems (especially with chemistries like LiFePO₄)
Even after hundreds of cycles, a well-maintained lithium-ion battery retains a high percentage of its original capacity — often 80% or more.This durability translates into lower long-term costs, less environmental waste, and more reliable performance over time.
Construction of Lithium-Ion Battery
Let’s explore the lithium-ion battery construction and working by first understanding the internal components of a typical cell.
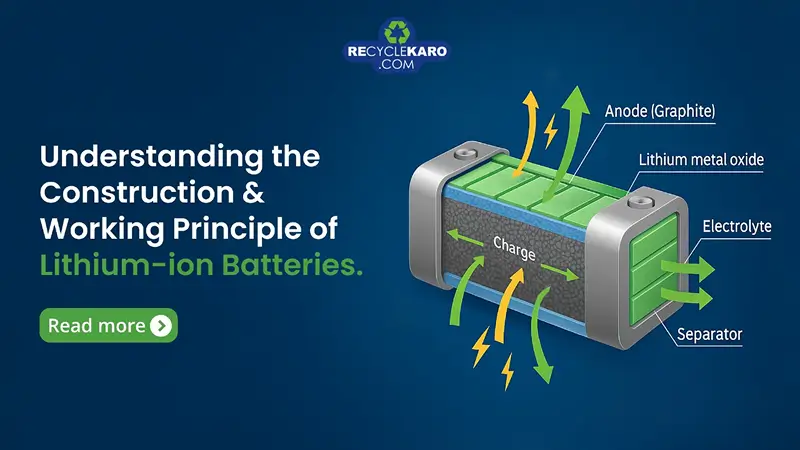
1. Anode (Negative Electrode)
- Usually made from graphite
- Stores lithium ions during the battery’s charged state
- Releases lithium ions during discharge
2. Cathode (Positive Electrode)
- Made from a lithium metal oxide such as lithium cobalt oxide (LiCoO₂), lithium iron phosphate (LiFePO₄), or lithium nickel manganese cobalt oxide (NMC)
- Accepts lithium ions during charge
- Releases lithium ions during discharge
3. Electrolyte
- A lithium salt (like LiPF₆) dissolved in an organic solvent
- Facilitates the movement of lithium ions between anode and cathode
4. Separator
- A porous membrane that prevents direct contact between anode and cathode
- Allows lithium ions to pass through during charge/discharge
5. Current Collectors
- Usually made of copper (anode) and aluminum (cathode)
- Collect and transfer electrons to and from the external circuit
These components are layered and wound (in cylindrical batteries) or stacked (in pouch/prismatic batteries) inside a casing, often with integrated Battery Management Systems (BMS) for safety and performance optimization.
Working Principle of Lithium-Ion Battery
Now that we’ve covered the construction, let’s understand the lithium-ion battery working principle.
Discharging (Powering a Device)
During discharge:
- Lithium ions move from the anode to the cathode through the electrolyte.
- Electrons flow through the external circuit, powering your device.
- This flow of ions and electrons generates the electrical current you use.
Charging (Storing Energy)
During charging:
- An external power source (like a lithium-ion battery charger) pushes lithium ions from the cathode back to the anode.
- Electrons also move back into the anode through the external circuit.
- Lithium ions intercalate (embed) into the graphite structure of the anode, storing energy.
This process can be repeated hundreds to thousands of times depending on the battery’s design and usage.
Lithium-Ion Battery Working Mechanism (Step-by-Step)
To truly grasp how lithium-ion batteries work, let’s break down the working mechanism in a simplified sequence:
Step 1: Initial State – Fully Charged Battery
In the fully charged state, the battery has just completed a charge cycle and is now primed to power a device. At this point:
- Lithium ions (Li⁺) are mostly embedded (intercalated) within the anode, which is typically made of graphite.
- The cathode (usually a lithium metal oxide, such as LiCoO₂ or LiFePO₄) has been depleted of some of its lithium ions, which have migrated to the anode during charging.
- The electrolyte maintains a stable environment for ion transport but does not conduct electrons.
- The separator ensures there’s no direct contact between anode and cathode, preventing short circuits.
In this state, the battery is like a compressed spring, full of potential energy, ready to be released as electrical power.
Step 2: Discharge Begins – Powering a Device
When the battery is connected to a device (e.g., phone, laptop, EV motor), the discharge process begins. This is when the battery releases stored chemical energy as usable electrical energy.
- The external circuit is completed when the device is turned on.
- Electrons in the anode begin to flow through the external circuit, powering your device.
- This electron flow is what actually provides electrical current to your gadgets or motors.
At this moment, the lithium ions are ready to travel in the opposite direction to maintain the balance of charge.
Step 3: Ion Movement – Anode to Cathode via Electrolyte
As electrons move through the external circuit, lithium ions (Li⁺) simultaneously begin to migrate from the anode to the cathode through the electrolyte.
- This movement is essential for maintaining electrical neutrality in the system.
- The ions pass through the porous separator, which acts like a sieve, allowing only ions (not electrons) to pass through.
- At the cathode, the lithium ions reintegrate into the cathode’s structure by reacting with the metal oxide material, typically forming lithium-metal-oxide compounds once again.
This dual flow — electrons through the circuit and lithium ions through the electrolyte — constitutes the essence of how lithium-ion batteries work during discharge.
Step 4: Electron Flow – Delivering Power
While lithium ions travel internally through the battery, electrons take a different path:
- Electrons exit the anode and travel through the external circuit to the cathode.
- On their journey, these electrons pass through your device’s internal components — powering screens, sensors, motors, lights, etc.
- At the cathode, the electrons reunite with the lithium ions to complete the electrochemical reaction.
This coordinated movement of electrons and ions creates a smooth and efficient flow of electricity that powers everything from a smartwatch to an electric car.
Step 5: Charging – Reversing the Reaction
Once the battery has discharged and the device needs recharging, you connect it to a lithium-ion battery charger. This charger supplies electrical energy in the reverse direction.
- The charger pushes electrons from the cathode back to the anode through the external circuit.
- Simultaneously, lithium ions move from the cathode to the anode through the electrolyte.
- At the anode, the lithium ions embed themselves into the graphite layers again, restoring the battery to its fully charged state.
This step is essentially the reverse of the discharge process, and it’s why lithium-ion batteries are rechargeable.
Step 6: Repeat – Ready for the Next Use
After a complete charge, the battery returns to its initial state, with lithium ions stored in the anode and electrons ready to flow on demand.
- The battery is now ready for another discharge cycle.
- This cycle of charging and discharging can be repeated hundreds to thousands of times, depending on the battery chemistry and usage conditions.
This repeatable process — efficient, reversible, and reliable — is what makes lithium-ion technology so indispensable in modern energy storage systems.
Types of Lithium-Ion Batteries
Depending on the cathode material, lithium-ion batteries can be categorized into:
- Lithium Cobalt Oxide (LiCoO₂): High energy density, common in smartphones/laptops.
- Lithium Iron Phosphate (LiFePO₄): Safe, thermally stable, used in EVs and solar applications.
- Lithium Manganese Oxide (LiMn₂O₄): Good thermal stability, used in medical devices.
- Lithium Nickel Manganese Cobalt Oxide (NMC): Balanced performance, ideal for power tools and electric vehicles.
- Lithium Nickel Cobalt Aluminum Oxide (NCA): High power, used by Tesla and other EV manufacturers.
How Do Lithium-Ion Batteries Work in Cars?
In electric vehicles (EVs), how lithium-ion batteries work becomes particularly fascinating due to the complexity and scale of their application. EVs are powered by large battery packs composed of thousands of lithium-ion cells connected in series and parallel to deliver the necessary voltage and capacity. When the driver accelerates, the vehicle’s motor controller draws current from the battery pack to power the electric motor. A critical component of this system is the Battery Management System (BMS), which constantly monitors and regulates the battery’s voltage, temperature, and state of charge to ensure safe and efficient operation.
Additionally, EVs utilize regenerative braking technology, which captures kinetic energy during deceleration and converts it back into electrical energy, recharging the battery and improving overall energy efficiency. This intelligent use of lithium-ion battery technology is what enables EVs to deliver both high performance and sustainability.
So if you’re wondering how do lithium-ion batteries work in cars, the principle is the same, but scaled up for greater power and efficiency.
How Do Lithium-Ion Batteries Work in Cars?
A lithium-ion battery charger plays a crucial role in the battery’s life and safety. Here’s the working principle of the charger:
- Constant Current Phase (CC): Delivers a steady current while voltage rises.
- Constant Voltage Phase (CV): Maintains a constant voltage while reducing current.
- Cut-off: Stops charging once full voltage is reached to avoid overcharging.
Chargers are usually designed to match the specific voltage and chemistry of the battery pack.
While lithium-ion batteries are efficient, improper handling can lead to issues like:
- Overcharging or deep discharging
- Thermal runaway
- Swelling or leakage
- Short circuits
This is why modern lithium-ion systems integrate Battery Management Systems (BMS) to monitor charge/discharge cycles, temperature, and cell balance.
Conclusion
Understanding the construction and working principle of lithium-ion batteries reveals the intricate science behind the power sources that fuel modern life. From smartphones to electric vehicles, the working of a lithium-ion battery is a marvel of chemistry and engineering. By mastering this knowledge, we can better appreciate the technology and make informed choices in our energy consumption.
Frequently Asked Questions (FAQs)
1. What is a lithium-ion battery and how does it work?
A lithium-ion battery is a rechargeable battery that works by the movement of lithium ions between anode and cathode through an electrolyte during charge and discharge cycles.
2. How do lithium-ion batteries work?
They operate on the principle of intercalation, where lithium ions shuttle back and forth between electrodes to store and release electrical energy.
3. What is the working principle of lithium-ion battery?
The working principle involves lithium ions moving from the anode to the cathode during discharge and back during charging, creating a flow of electrons in the external circuit.
4. How does a lithium-ion battery charger work?
It charges the battery in two phases: constant current and constant voltage, ensuring safe charging by regulating power input.
5. How do lithium-ion batteries work in cars?
EVs use large lithium-ion battery packs to power electric motors, controlled by advanced systems for thermal management, charge control, and energy recovery via regenerative braking.
6. What are the main components of a lithium-ion battery?
The main parts include anode, cathode, electrolyte, separator, and current collectors.
7. Are lithium-ion batteries safe?
Yes, when properly manufactured and used with integrated safety systems. Risks arise only when batteries are overcharged, punctured, or exposed to extreme conditions.
8. Can lithium-ion batteries be recycled?
Yes, they can be recycled to recover valuable materials like lithium, cobalt, and nickel, though recycling infrastructure is still developing in many regions.





You can find all the useful information you need on the Vavada mirror. Find out why players switch to the official Vavada website via the mirror, and discover the benefits: similar registration and login processes, bonuses, promo codes